- Full visibility and control over all processes
- Real-time business insights
- Simplify complex operational workflows
- Robust tracking and traceability tools
- Accountability with integrated financials
- Tight control over banking and cash flow
MedTech redefined
Designed specifically for medical device manufacturers, Priority's cloud ERP solution delivers a trusted, market-proven solution to help meet the challenges of R&D, engineering, rigorous QA cycles, and stringent regulatory standards and requirements. Priority ERP can help you complete critical operational tasks, including lot tracking, inventory costing, and planning and forecasting, while the system’s integrated reporting and analysis tools give you all the information you need to make better-informed business decisions.
Complexity simplified
From complex manufacturing processes, supported by robust tools to manage financials, track and trace, product identification and serialization, warranty and service tracking, and more, Priority’s medical device ERP software helps you deliver precision-quality, fully-compliant products – all on a single platform.

Precision production and quality assurance is our priority
Medical Devices ERP Key features
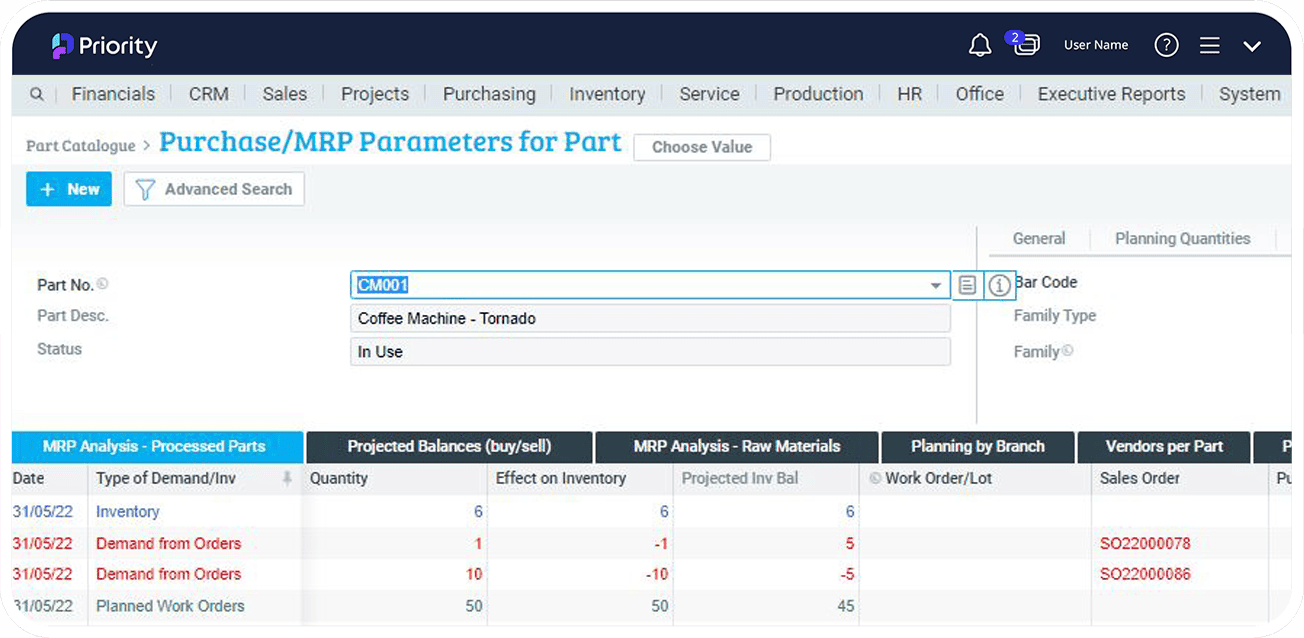
Material Requirements Planning (MRP)
A robust production planning, scheduling, and inventory control system to manage your manufacturing processes, Priority MRP determines material requirements based on sales orders, open work orders or frequency of need. Integrated into Priority ERP, MRP helps you effectively plan your manufacturing tasks, delivery schedules, purchasing activities, and more..
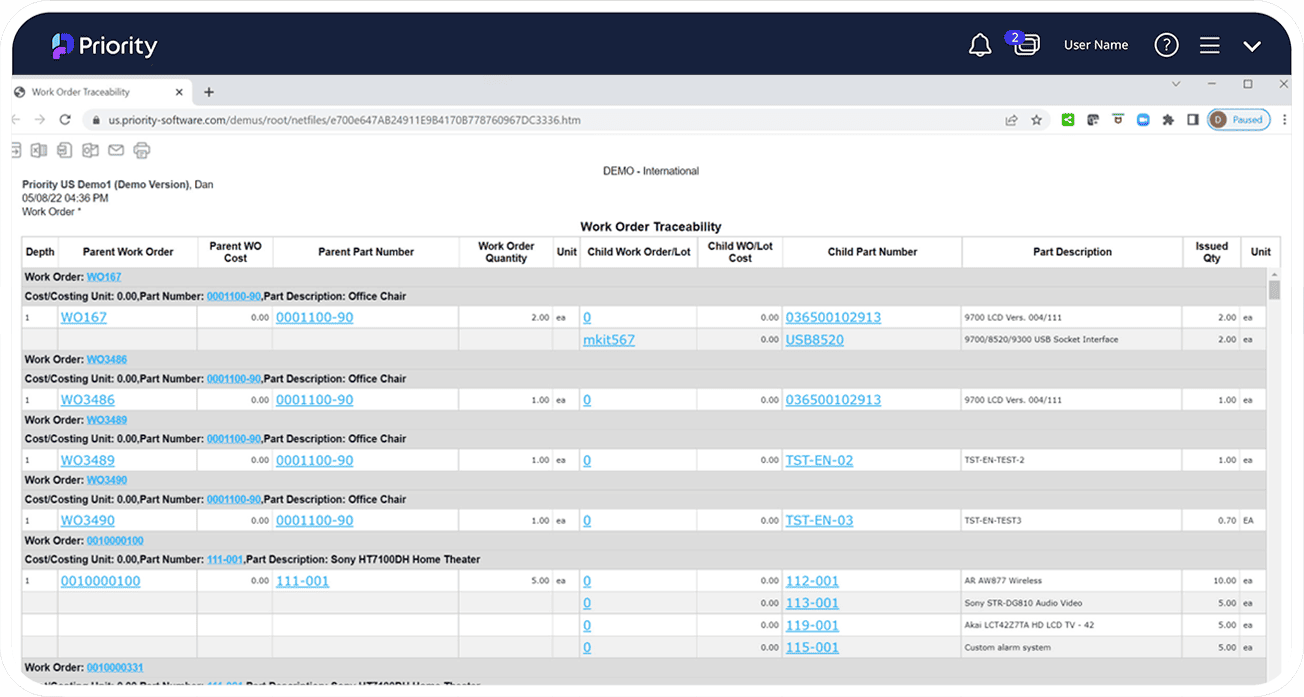
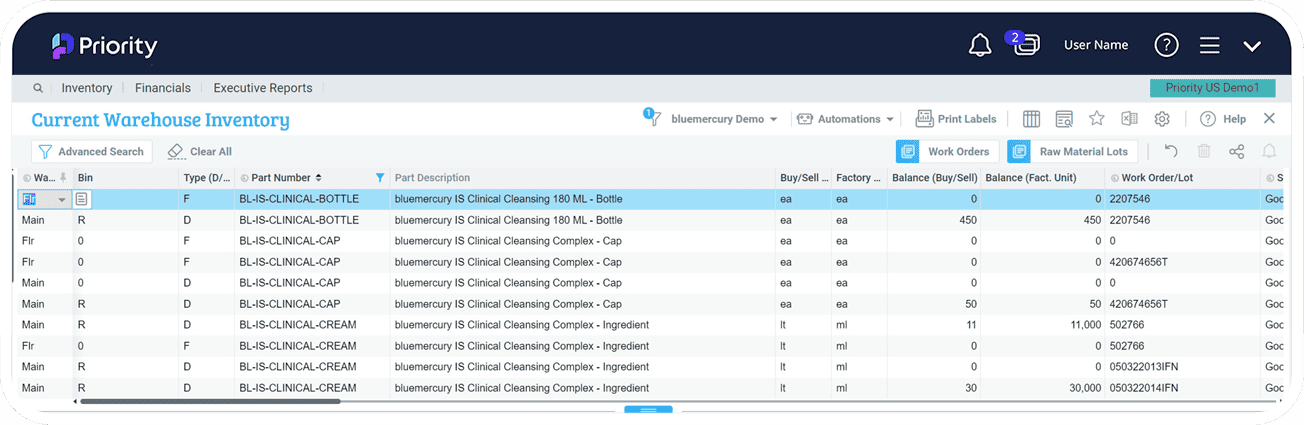
Serial, Lot Tracking and Traceability
Priority’s Serial and Lot Tracking ensures reliable management of material and product traceability through every stage in your supply chain, from receipt of raw materials, through delivery to your end-customer. You’ll have full control over parts as they move through your production process, and track serialized parts and their lot attributes throughout the entire part lifecycle.
Inventory Control
The cornerstone of any successful manufacturing operation, inventory management and control is what drives your shop floor, supports materials handling, and helps increase your revenues. Maintain optimum inventory levels by inventory analysis, save on costs and resources, improve service levels to meet current and future production needs, and manage tracking and traceability, with tight control on inventory replenishment.
Product Data Management (PDM)
PDM is the process of capturing and managing your organization’s product-related data, so it can be reused in business processes, such as sales order management, purchasing, cost accounting, and logistics. Part of the greater picture, Product Lifecycle Management (PLM), PDM also maintains your manufacturing part numbers, Bill of Materials (BOM), and integrates with Product Change Control (PCC).
RoHS Compliance
Priority ERP’s inventory management module is fully compliant with the Restriction of the Use of Certain Hazardous Substances in Electronic Equipment (RoHS) Directive, a mandatory requirement for the continued sales of medical device equipment, and general electrical products and components.
Cost Management
With a wide range of integrated cost management tools, Priority provides full support for FIFO and Moving Average costing methods, including flexible costing parameter definition, cost burdening, and the ability to make calculations based on standard or actual costs, to increase profitability, and your bottom line.
QA Sampling and Lab Testing
Ensure that your manufacturing processes adhere to the correct procedures, use required equipment, and comply with relevant industry regulations and standards. Priority ERP enables you to view, access and share QA sampling and lab testing data, including worklists, due dates, testing methods, or inspection lot details. Full visibility of real-time validated information, leads to better-informed and more accurate decision-making.