- Gain real-time visibility into your supply chain, allowing you to track inventory levels, monitor orders, and optimize production
- Comply with local and international regulatory requirements for safety, quality, and traceability throughout the product life cycle
- Manage finances and accounting accurately with financial reports, budgeting, and forecasting tools to help you make informed decisions and optimize cash flow
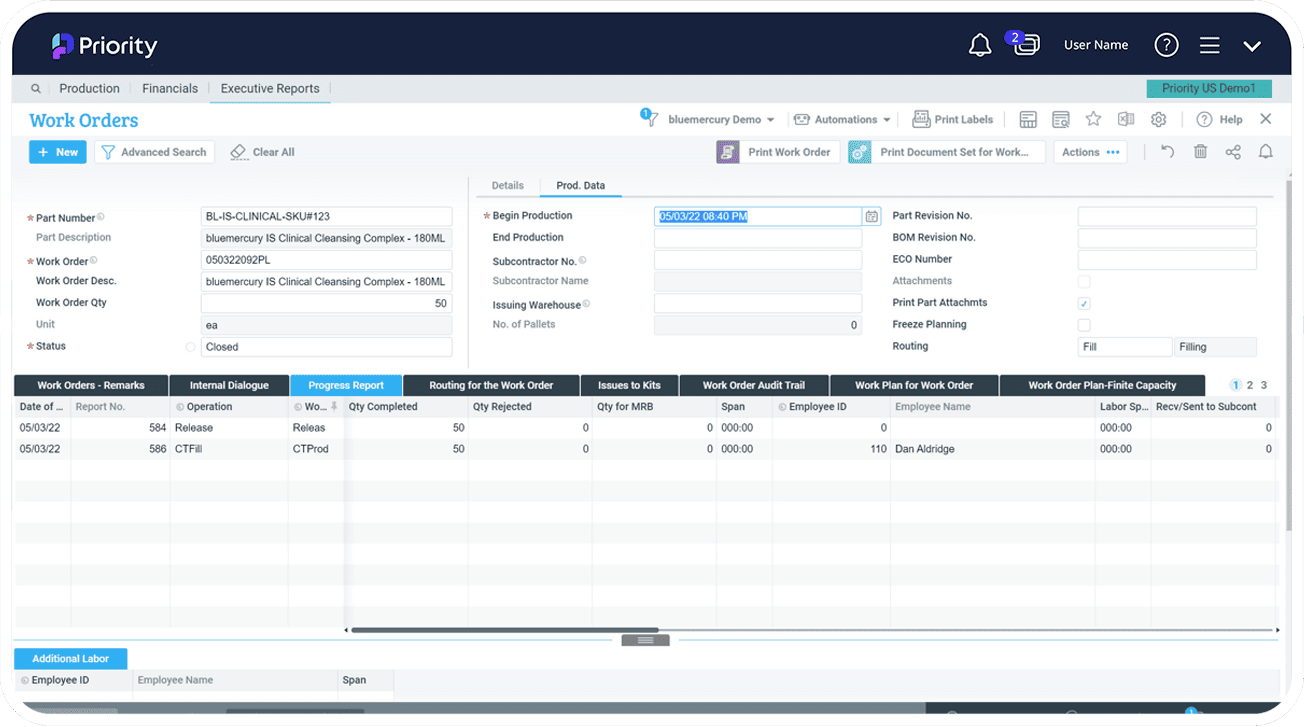
- Trace every component of your products throughout the supply chain, from raw materials to finished products, and monitor all process steps for full traceability.
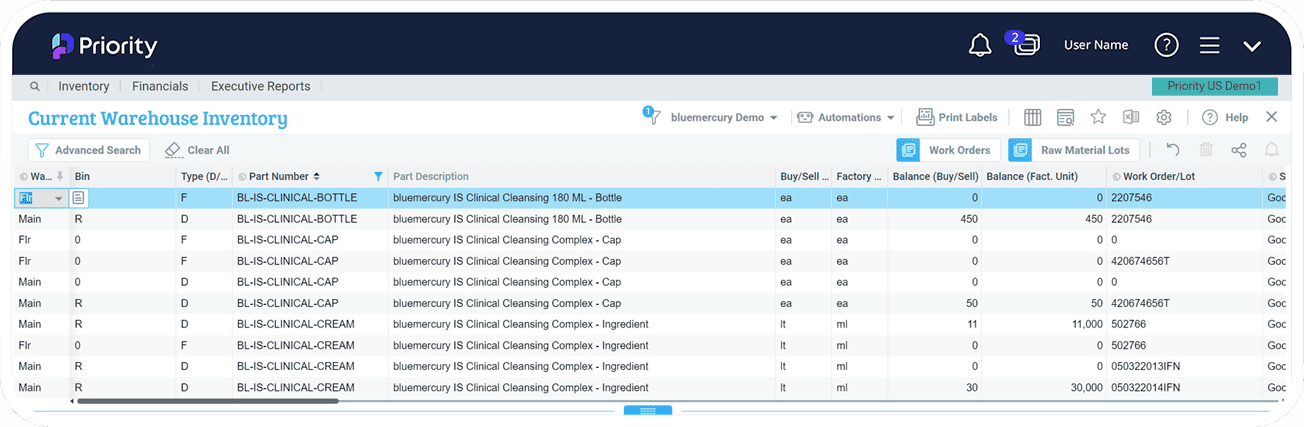
- Track real-time inventory levels, expiration dates, and batch numbers to reduce waste, increase efficiency, and optimize inventory levels.
- Gain insights into customer needs and preferences and target them with customized promotions and marketing campaigns.
Prescription for success
Pharmaceutical companies are highly regulated and require strict control over their operations, from research and development to manufacturing and distribution. Priority's ERP software for pharmaceuticals provides a comprehensive suite of tools and features to help manage these complex operations, including quality management, batch tracking, compliance with regulatory standards, and supply chain management. Priority's cloud-based ERP enables end-to-end supply chain management, tracking and tracing of products, optimized inventory management, and real-time data analysis for more informed decision-making.
Raising the bar on pharmaceutical product manufacturing
With an advanced set of tools to support fundamental processes in development, sales and distribution, including R&D project management, process manufacturing, work order and document management, MRP, traceability, QA, lab management, and Material Review Board (MRB), Priority’s pharmaceutical ERP software enables informed decisions, improved efficiency, and ensures compliance while reducing costs and enhancing the overall product quality.
Multiple distribution channels made easy
Priority cloud ERP manages various distribution models: wholesalers that purchase your inventory for distribution or sell and distribute products on your behalf directly to healthcare professionals; hospitals or pharmacy chains. With Priority cloud ERP for Pharma, you can store finished products, manage and control stock movement, and track and trace your products in partnership with CMOs, third-party logistics providers, and wholesalers.

Get the right dose of efficiency
Pharmaceutical ERP Software Key Features
WMS
Priority’s robust Warehouse Management System optimizes inventory service levels, and boosts warehouse efficiency, by managing and controlling the storage and movement of your inventory. With Priority WMS, you’ll gain full control of your entire operational process, from the time your products enter your warehouse, until they move out, including inventory management, picking, auditing, and more.
Distribution and Delivery Tracking
Priority ERP integrates with leading courier companies, such as ShipEngine, the multi-carrier shipping platform that powers ShipStation, a web-based e-commerce shipping solution that works seamlessly with internal and external warehouses, whereby the system is able to limit sales of specific products to specific countries, including managing stock in consignment.
Material Review Board (MRB)
Priority’s Material Review Board module enables you to electronically document, manage and track discrepancies with materials, whether raw materials, work in process, or finished goods, to efficiently record investigative checks done on failed components or processes, and improve overall product quality.
Multiple Units of Measure
Priority supports multiple Units of Measure (UoMs) in a single item, and a fully-automated conversion process between various UoMs, converting all quantities entered in other units to the base unit of measure, including measures by weight, volume or quantity, and manufacturer and factory units.
Inventory Control
Manage your inventory levels and maintain tight control on inventory replenishment. Seamlessly integrated into Priority ERP, you’ll have a handle on your business planning and operations, by managing all of your finances, logistics, and BoM data, including version control per product, make-to-stock and make-to-order, with full support of FIFO and product expiration dates.
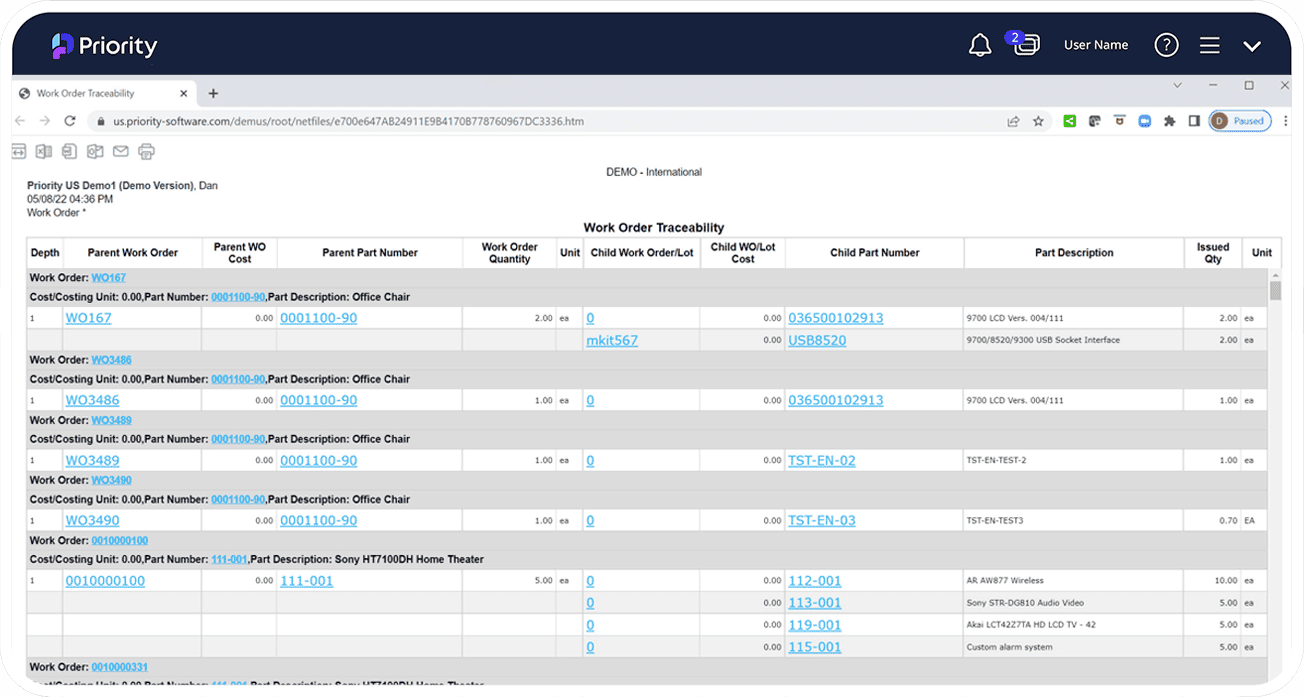
Tracking and Traceability
Track expiration dates on products and raw materials, through packing, warehousing, and on to final delivery, including the ability to maintain GPS coordinates to identify the product origin. Easily track and trace the product, or access product information along the supply chain via Priority ERP’s recorded identification via barcode or RFID tag.
Case Studies
Pharmaceutical ERP FAQ’s
How is ERP used in Pharma companies?
ERP systems are widely used in the pharma industry to manage and automate various business processes such as production planning, inventory management, quality control, batch management, regulatory compliance, and financial management. An ERP system allows companies to integrate these processes into a single platform, improving operational efficiency and visibility, reducing errors and delays, and facilitating compliance with industry standards and regulations. Additionally, ERP systems can help pharma companies track the full life cycle of their products, from development to distribution and post-market surveillance, allowing them to make data-driven decisions and improve patient outcomes.
What are the advantages of using an ERP in Pharma?
The advantages of using an ERP system in the pharma industry are numerous. An ERP system provides end-to-end management of business processes such as production planning, quality control, supply chain management, and compliance management. With an integrated system, companies can easily track and monitor raw materials, inventory levels, and shipments, ensuring that the right products are delivered to customers at the right time. Additionally, an ERP system can help pharma companies maintain compliance with regulatory requirements, enabling them to meet stringent quality standards and reduce the risk of costly fines and product recalls. With real-time data and analytics, companies can make informed decisions about their operations, improve collaboration and communication, and ultimately enhance their bottom line.
What are the key modules for Pharma companies?
Pharma companies require key modules in an ERP system to manage their operations efficiently, including inventory management, batch and lot management, quality control management, compliance management, manufacturing management, and financial management. An ERP system helps to ensure adherence to regulatory requirements and industry standards, track critical data and metrics, streamline production processes, optimize inventory levels, and improve decision-making capabilities. Additionally, an integrated CRM module can help pharma companies manage customer relationships and track interactions with healthcare providers, patients, and distributors.
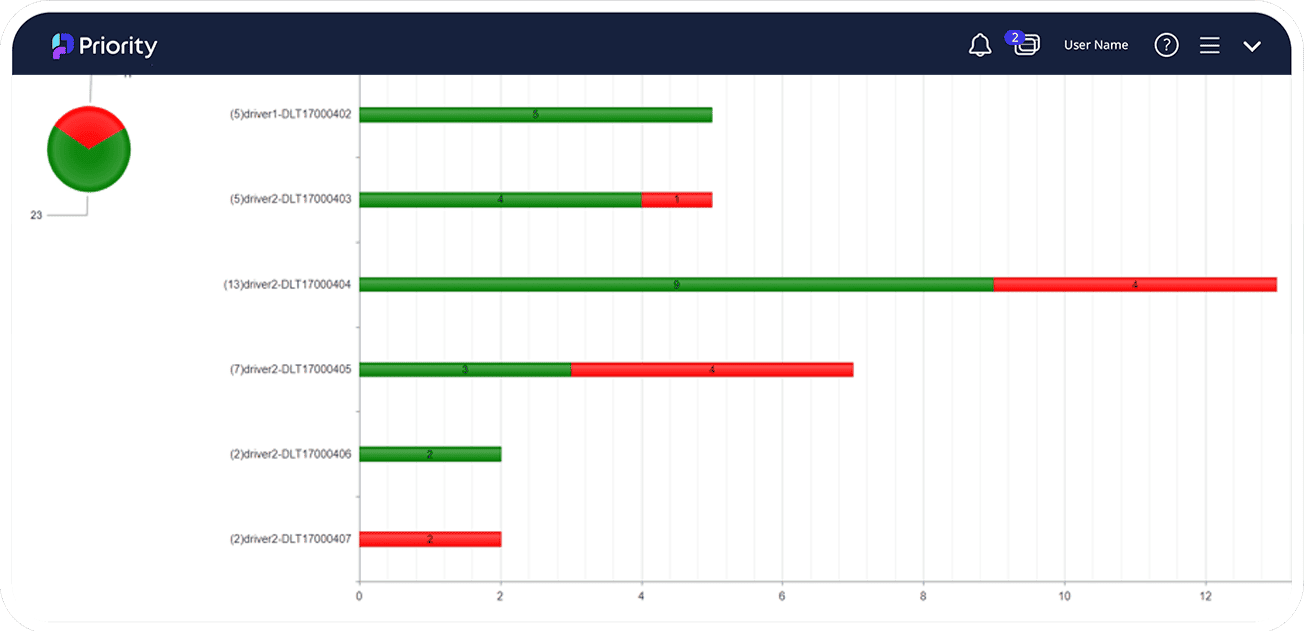
How does an ERP system improve recall management in the Pharma industry?
An ERP system can improve recall management in the pharmaceutical industry by providing real-time tracking and traceability of products throughout the supply chain, enabling companies to quickly and accurately identify affected products and their location, initiate a recall, and notify customers and regulatory authorities as needed. With an ERP system, companies can efficiently manage the recall process, minimize the impact on customers and the company's reputation, and comply with regulatory requirements. The system can also help identify the root cause of the issue, enabling companies to take corrective action to prevent future recalls.